The additivity equations used in this system are described below.
The temperature or viscosity must be specified as an input condition for certain additivity equations. A special condition is one in which the calculation is limited by the relationship between two or more compositions, even if each component fulfils the conditions shown in the [Condition of Eq.] column in the [Additivity Equation] window.
The following expressions are used for composition ratios.
pi: mol%
xi: mass%
ci: cationic mol%
Note: Specify SiO2 as one of the component when [Silicate] is clearly described.
(1) Density
| 1) | Toge, Tanaka and Minami (Si-As-Te Chalcogenide) Input condition: None Special condition: None Source: N. Toge, T. Minami, M. Tanaka, J. Am. Ceram. Soc., 59, 461 (1976) |
| 2) | Toge, Tanaka and Minami (Ge-As-Te Chalcogenide) Input condition: None Special condition: None Source: N. Toge, T, Minami, M. Tanaka, J. Am. Ceram. Soc., 59, 461 (1976) |
| 3) | Toge, Tanaka and Minami (As-Te-Se Chalcogenide) Input condition: None Special condition: None Source: N. Toge, T, Minami, M. Tanaka, J. Am. Ceram. Soc., 59, 461 (1976) |
| 4) | Toge, Tanaka and Minami (Ge-Te-Se Chalcogenide) Input condition: None Special condition: None Source: N. Toge, T, Minami, M. Tanaka, J. Am. Ceram. Soc., 59, 461 (1976) |
| 5) | Tanaka and Minami (As-S Chalcogenide) Input condition: None Special condition: It is possible to calculate at 0 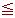 As As 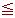 64.1mass%. 64.1mass%.Source: M. Tanaka, T. Minami, Jpn. J. Appl. Phys., 4, 939 (1965) |
| 6) | Appen (Silicate) Input condition: None Special condition: Refer to souece. Source: A. A. Appen, Kimiya Stekla, Leningrad (1974) |
| 7) | Gan, Fuxi (Fluoride) Input condition: None Special condition: None Source: F. Gan, J. Non-Cryst. Solids, 184, 9 (1995) |
| 8) | Huggins (Silicate) Input condition: None Special condition: Please refer to source. Source: M. L. Huggins, K-H. Sun, J. Am. Ceram. Soc., 26, 4 (1941) |
| 1) | Inaba, Fujino and Morinaga (Silicate) Input condition: None Special condition: The density is calculated by the equation of Huggins (Silicate). Source: S. Inaba, S. Fujino, K. Morinaga, J. Am. Ceram. Soc., 82, 3501 (1999) |
| 2) | Makishima and Mackenzie Input condition: None Special condition: The density is calculated by the equation of Huggins (Silicate). Source: A. Makishima, J. D. Mackenzie, J. Non-Cryst. Solids, 12, 35 (1973) |
| 1) | Dietzel (Silicate) Input condition: None Special condition: The equation is effective for a temperature of 900  . . Source: A. Dietzel, Sprechsaal, 75, 82 (1942) |
| 2) | Lyon (Silicate) Input condition: None Special condition: Equation is effective for a temperature of 1200  Source: K. C. Lyon, J. Am. Ceram. Soc., 27, 186 (1944) |
| 3) | Appen (Silicate) Input condition: None Special condition: Equation is effective for a temperature of 1300  . At . At 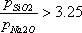 , it is possible to calculate. , it is possible to calculate. Source: A. A. Appen, Silikattechn, 5, 11 (1954) |
| 4) | Sasek (Silicate) Input condition: Input temperature of 1200  or 1400 or 1400 . . Special condition: None Source: L. Sasek, M. Houser, Chem. Technol. Silik., L5, 49 (1974) |
| 1) | Appen (Silicate) Input condition: None Special condition: It is possible to calculate for compositions containing TiO2 only at 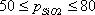 . . Source: A. A. Appen, Kimiya Stekla, Leningrad 1974 |
| 2) | Winkelmann and Schott (Silicate) Input condition: None Special condition: Equation is effective in the temperature range 20 - 100  . .Source: A. Winkelmann, O. Schott, Ann. Physik, 51,735 (1894) |
| 3) | Takahashi (0 - 100 ) (Silicate) ) (Silicate)Input condition: None Special condition: It is possible to calculate at 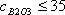 cationic mol%. cationic mol%. Source: K. Takahashi, J. Ceram. Soc. Japan, 63, 142 (1955) |
| 4) | Takahashi (0 - 400 ) (Silicate) ) (Silicate)Input condition: None Special condition: It is possible to calculate at 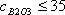 cationic mol%. cationic mol%. Source: K. Takahashi, J. Ceram. Soc. Japan, 63, 142 (1955) |
| 5) | Tanaka and Minami (As-S Chalcogenide) Input condition: None Special condition: It is possible to calculate at 0 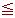 As As 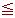 43.32mol%. 43.32mol%.Source: M. Tanaka, T. Minami, M. Hattori, Jpn. J Appl. Phys., 5, 185 (1966) |
| 1) | Ratcliffe (Silicate) Input condition: Input temperature of -100  , 0 , 0 or 100 or 100 . .Special condition: None Source: E. H. Ratcliffe, Glass Technol., 4, 113 (1963) |
| 2) | Russ (Silicate) Input condition: None Special condition: Equation effective for 0  . .Source: A. Russ, Sprechsaal, 61, 887 (1921) |
(6) Specific Heat
| 1) | Sharp and Ginther (mean over 0 - t  ) (Silicate) ) (Silicate)Input condition: Input the temperature (range 0 - 1300  ). ). Special condition: It is possible to calculate for compositions containing Mn3O4 and Fe2O3 only for temperatures lower than 600  . . Source: D. E. Sharp, L. B. Ginther, and J. Am. Ceram. Soc., 34, 260 (1951) |
| 2) | Sharp and Moore (Silicate) Input condition: Input the temperature (range 0 - 1300  ). ). Special condition: It is possible to calculate for compositions containing Mn3O4 and Fe2O3 only for temperatures lower than 600  . . Source: J. Moore, D. E. Sharp, and J. Am. Ceram. Soc., 41, 461 (1958) |
| 3) | Schwiete and Ziegler (Silicate) The input condition: Input the temperature (range 0 - 1300  ). ). Special condition: It is possible to calculate for compositions containing Mn3O4 and Fe2O3 only for temperatures lower than 600  . . Source: H. E. Schwiete, G. Ziegler, and Glastechn. Ber., 28, 137 (1955) |
| 1) | Lakatos (1978) (Silicate) Input condition: Input the viscosity as 102.5, 103.5 or 104.5 dPa·s. Special condition: It is possible to calculate at 52 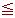 SiO2 SiO2 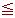 62.2mass%. 62.2mass%.Source: T. Lakatos, L.-G. Johansson, B. Simminingskold, Glasteknisk Tidskrift, 33, 55-59 (1978) |
| 2) | Lakatos (1979) (Silicate) Input condition: Input the viscosity as 102, 104 or 106 dPa·s. Special condition: It is possible to calculate at 57 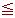 SiO2 SiO2 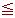 74mass%. 74mass%.Source: T. Lakatos, L.-G. Johansson, B. Simminingskold, Glasteknisk Tidskrift, 34, 61-65 (1979) |
| 3) | Okhotin (SiO2-Na2O-CaO) Input condition: Input the viscosity (range103 - 1013 dPa·s). Special condition: None Source: M. V. Okhotin, Steklo i Keramika, 11, 1 (1954) |
| 4) | Sasek (SiO2-Na2O-CaO-MgO) Input condition: None Special condition: Equation effective for a viscosity of 1013 dPa·s. Source: L. Sasek, Silikaty, 16, 207 (1973) |
(8) Transition Temperature
| 1) | Tanaka and Minami (As-S Chalcogenide) Input condition: None Special condition: It is possible to calculate at 0 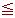 As As 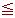 43.32mol%. 43.32mol%.Source: M. Tanaka, T. Minami, M. Hattori, Jpn. J Appl. Phys., 5, 185 (1966) |
| 1) | Lakatos(1975) (Silicate) Input condition: Input the temperature. Special condition: Equation effective within the viscosity range 102 - 106 (dPa·s). Source: T. Lakatos, L.-G. Johansson, B. Simminingskold, Glasteknisk Tidskrift, 30, 7-8 (1975) |
| 2) | Lakatos (1976) (Silicate) Input condition: Input the temperature. Special condition: Equation effective within the viscosity range 102 - 106 (dPa·s). Source: T. Lakatos, Glasteknisk Tidskrift, 31, and 51-54 (1976) |
| 3) | Sasek (Silicate) Input condition: Input the temperature. Special condition: Equation effective within the viscosity range 102.3 - 104 (dPa·s). Source: L. Sasek, Silikaty, 16, 207(1973) |
| 4) | Hrma (Silicate) Input condition: Input the temperature (range 950 - 1250  ). ). Special condition: None Source: P. Hrma, R. J. Robertus, Ceram. Eng. Sci. Proc., 14, 187 (1993) |
| 5) | Urbain (Silicate) Input condition: Input the temperature. Special condition: None Source: G. Urbain, F. Cambier, M. Deletter, M. R. Anseau, Trans. J. Br. Ceram. Soc., 80, 139 (1981) |
| 1) | Appen (Silicate) Input condition: None Special condition: Complex condition (Refer to source) Source: A. A. Appen, Kimiya Stekla, Leningrad (1974) |
| 2) | Gan Fuxi (Fluoride) Input condition: None Special condition: None Source: F. Gan, J. Non-Cryst. Solids, 184, 9 (1995) |
| 3) | Huggins (Silicate) Input condition: None Special condition: Refer to source. Source: M. L. Huggins, Sun K-H., J. Am. Ceram. Soc., 26, 4 (1941) |
(11) Abbe's Number
| 1) | Appen (Silicate) Input condition: None Special condition: Refer to source. Source: A. A. Appen, Kimiya Stekla, Leningrad (1974) |
| 2) | Gan Fuxi (Fluoride) Input condition: None Special condition: None Source: F. Gan, J. Non-Cryst. Solids, 184, 9 (1995) |
| 3) | Huggins (Silicate) Input condition: None Special condition: Refer to source. Source: M. L. Huggins, K-H. Sun, J. Am. Ceram. Soc., 26, 4 (1941) |
(12) Mean Dispersion
| 1) | Appen (Silicate) Input condition: None Special condition: Refer to source. Source: A. A. Appen, Kimiya Stekla, Leningrad (1974) |
| 2) | Gan Fuxi (Fluoride) Input condition: None Special condition: None Source: F. Gan, J. Non-Cryst. Solids, 184, 9 (1995) |
| 3) | Huggins (Silicate) Input condition: None Special condition: Refer to source. Source: M. L. Huggins, K-H. Sun, J. Am. Ceram. Soc., 26, 4 (1941) |
| 1) | Sasek and M. (High Temp.) (Silicate) Input condition: Input the temperature (range 1000 - 1400  ). ). Special condition: None Source: L. Sasek, H. Meissnerova, Technol. Silik., L5, 111 (1974) |
| 2) | Sasek and M. (Low Temp.) (Silicate) Input condition: Input the temperature (range 320 - 540  ). ). Special condition: None Source: L. Sasek, H. Meissnerova, Technol. Silik., L5, 111 (1974) |
| 3) | Hrma (Silicate) Input condition: Input the temperature (range 950 - 1250  ). ). Special condition: None Source: P. Hrma, R. J. Robertus, Ceram. Eng. Sci. Proc., 14, 187 (1993) |
(14) DC Resistivity
| 1) | Mazurin (Silicate) Input condition: Input the temperature (range 100 - 427  ). ). Special condition: SiO2:20-80, B2O3:0-10, Al2O3:0-10, Na2O+K2O:12-30, RO=MgO+CaO+BaO+ZnO+PbO:1-20mol%. However when CaO or BaO exist, singly or together in RO, RO=CaO+BaO:1-28mol% Source: O. V. Mazurin, The structure of glass, Vol. 4: Electrical conductivity and structure of glass, New York. Consultant Bureau (1965) |
The coefficients, activation energy, etc. of the property equations are included as INTERGLAD data. The equations consist of six expressions, as below. When the [Figure] pull-down menu of the detail window is clicked for a glass for which the coefficient data of these equations is complete, the relation between the property value and temperature or wavelength is displayed as a figure.
The property equations are shown below. The numerical values in parentheses denote the ID No. of the property.
(1) Fulcher's equation
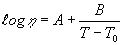
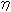 : Viscosity dPa.s
: Viscosity dPa.sA : Coefficient of Fulcher's equation (1231) log(dPa.s)
B : Coefficient of Fulcher's equation (1232) C
T0: Coefficient of Fulcher's equation (1233) C
T : Temperature C
(2) Diffusion Formula
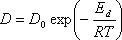
D : Diffusion coefficient m2/s
R : Gas constant 8.314 J.K-1mol-1
D0 : Diffusion coefficient factor(1301,1303,-,1363) m2/s
Ed : Activation energy(1302,1304,-,1364) J.mol-1
T : Temperature K
(3) Dispersion Formula
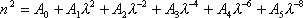
n : Refractive index
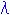 : Wavelength 0.25-1.55
: Wavelength 0.25-1.55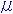 m
mA0 - A5 : constant of dispersion formula (2101-2106)
(4) Electric Conductivity Formula
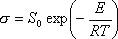
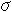 : Electric Conductivity S/m
: Electric Conductivity S/mR : Gas constant 8.314 J.K-1mol-1
S0 : Coefficient of Formula (3041) S/m
E : Activation energy (3045) J.mol-1
T : Temperature K
(5) DC Volume Resistivity Formula
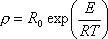
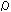 : DC Volume Resistivity Ohm.m
: DC Volume Resistivity Ohm.mR : Gas constant 8.314 J.K-1mol-1
R0 : Coefficient of Formula (3077) Ohm.m
E : Activation energy (3078) J.mol-1
T : Temperature K
(6) AC Volume Resistivity Formula
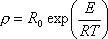
 : AC Volume Resistivity Ohm.m
: AC Volume Resistivity Ohm.mR : Gas constant 8.314 J.K-1mol-1
R0 : Coefficient of Formula (3085) Ohm.m
E : Activation energy (3086) J.mol-1
T : Temperature K
The properties which can be shown in a figure are given below. The values of these properties can be maintained as table data, and displayed by clicking the [Figure] pull-down menu of the detail.
The number to the right of each property is the property ID.
- Thermal expansion curve (α -T Curve) 1040
- UV-IR Transmission Spectrum 2218
- UV-IR Absorption Spectrum 2278
- UV-IR Reflectance Spectrum 2398
- Emission Spectrum 2509
The table data format is as follows in a CSV file.
| 1st line : | Title of figure |
| 2nd line: | Label name (unit) for X-axis, minimum value, maximum value |
| 3rd line: | Label name (unit) for Y-axis, minimum value, maximum value |
| 4th line: | Data (X11,Y11) |
| ....... | |
| n+3th line: | Data (X1n,Y1n) |
| n+4th line: | Data (X21,Y21) |
| ....... | |
| 2n+3 th line: | Data (X2n,Y2n) |
| ....... |
where, X1* and X2* are other curves. A figure can display two or more curves.
(2) File names for table data
The file names for table data are assigned as follows:
expa_<glass code>.csv : Thermal expansion curve
trns_<glass code>.csv : UV-IR transmission spectrum
abso_<glass code>.csv : UV-IR absorption spectrum
refl_<glass code>.csv : UV-IR reflectance spectrum
emis_<glass code>.csv : Emission spectrum
(3) Storage of table data
Table data files are stored in the following folder (within the system application folder):
…\ngf\INTERGLAD5\figure
The glass-forming region can be displayed for the boundaries in addition to glasses denoted by "o" and "x". The data are input with the table data format. The format is in CSV as follows:
1st line : Unit of composition: 11 or 12 is input respectively as mass% or mol%.
2nd line: ID number of component A, component B and component C
3th line: Data (A1, B1, C1): as triangular coordinates.
.................
n+2th line: Data (An, Bn, Cn)
..................
Each points are connected by the smoothing function in a curve. Therefore, even if it is a little point, it is drawn as a smooth curve.